
Cristal History 1988 – The company incorporated 1991 – The first pigment was produced 1992 – Fully operated with 4 grades at 48,000 tpa 2000 – CRISTAL. - ppt download

Nanomaterials | Free Full-Text | Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution

Novel Method for Preparing Amorphous Titanyl Sulfate from Hydrous Titanium Oxide | Industrial & Engineering Chemistry Research
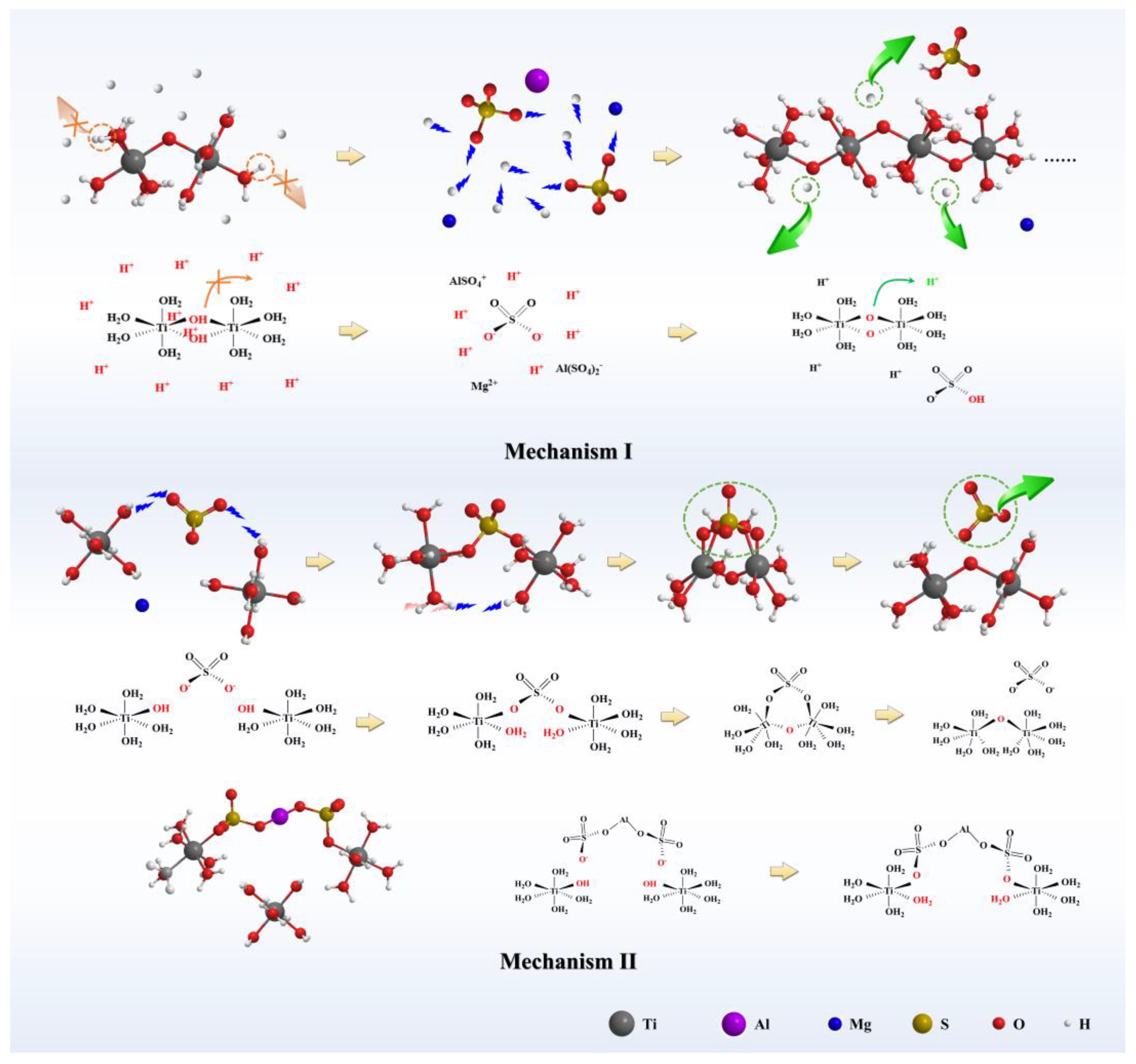
Nanomaterials | Free Full-Text | Preparation of Hydrated TiO2 Particles by Hydrothermal Hydrolysis of Mg/Al-Bearing TiOSO4 Solution

Processes | Free Full-Text | Mechanism, Thermodynamics and Kinetics of Rutile Leaching Process by Sulfuric Acid Reactions

Titanium(IV) oxysulfate Solution 1.9-2.1, For determination of hydrogen peroxyde (H 15), 1.9-2.1%, MilliporeSigma™ Supelco™ | Fisher Scientific

PDF) Effect of TiOSO 4 hydrothermal hydrolysis conditions on TiO 2 morphology and gas-phase oxidative activity | Alexander Vorontsov - Academia.edu

Supramolecular self-assembly synthesis of ordered mesoporous TiO2 from industrial TiOSO4 solution and its photocatalytic activities - ScienceDirect

Clean, low-cost, and efficient nano-TiO2 composite photocatalyst: Preparation and performance in TiOSO4 hydrolysis system - ScienceDirect
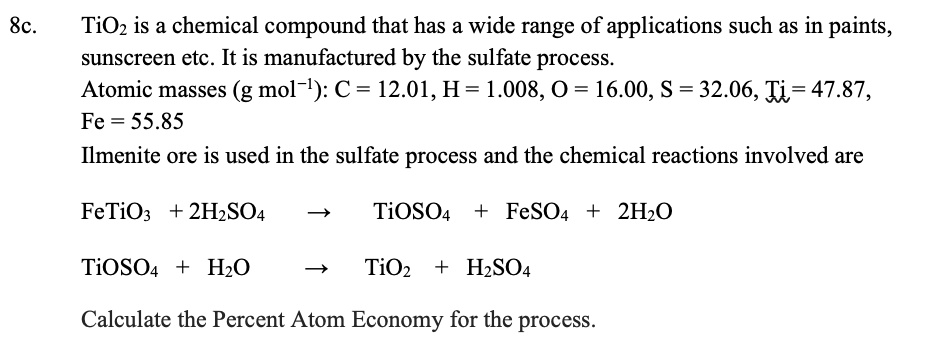
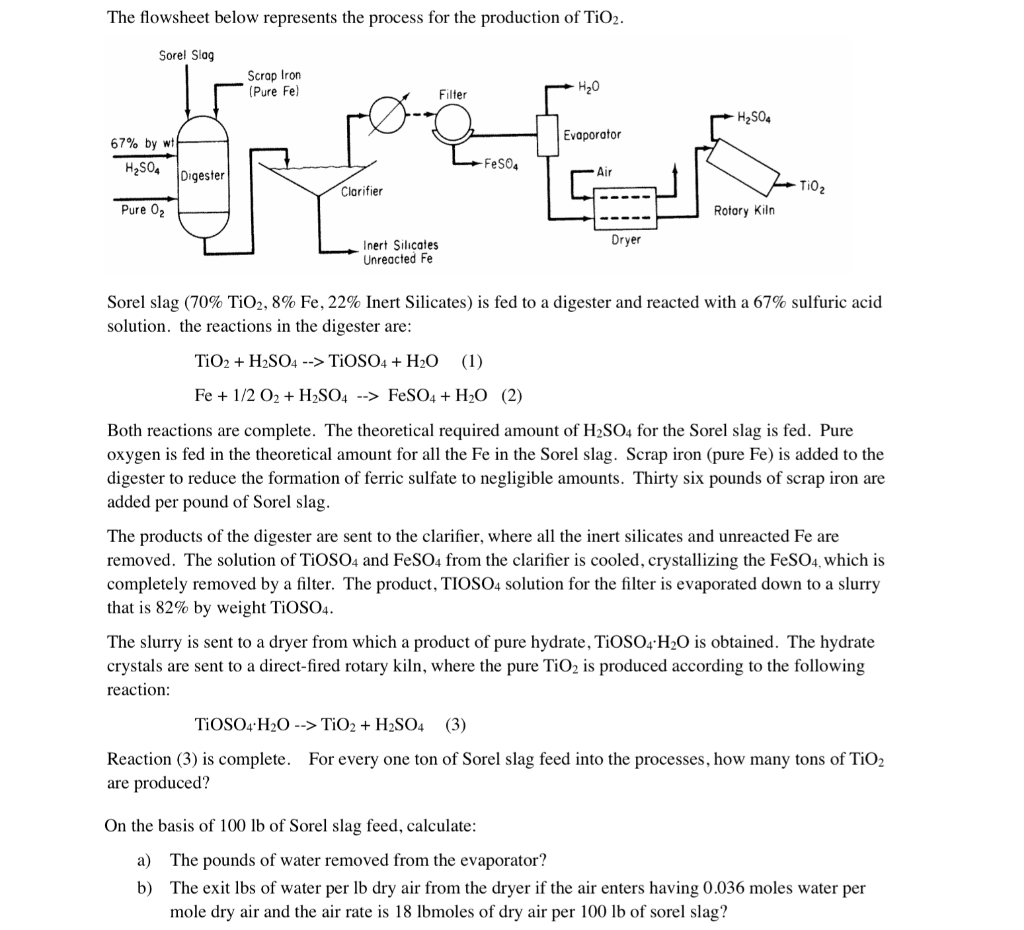
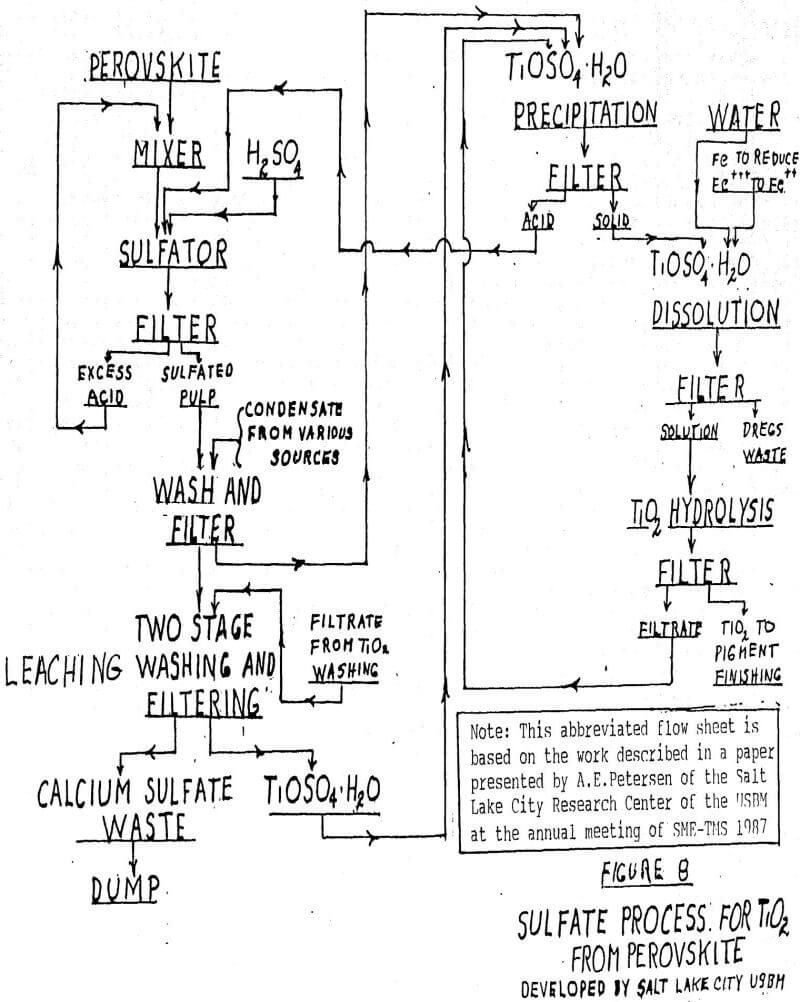





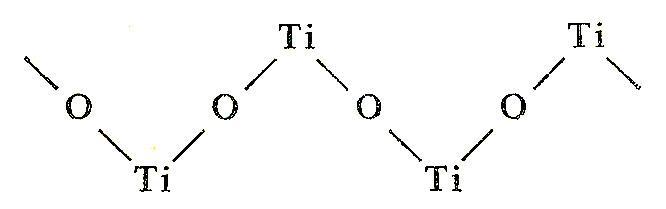

![PDF] Influence of hydrolysis in sulfate process on titania pigment producing | Semantic Scholar PDF] Influence of hydrolysis in sulfate process on titania pigment producing | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/f51fdcb711813023bae94e22833f7e56d341cbd6/3-Table2-1.png)