FDA Advisory No.2023-2526 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
FDA Advisory No. 2020-1348 || Public Health Warning Against the Purchase and Use of the following Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0621 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

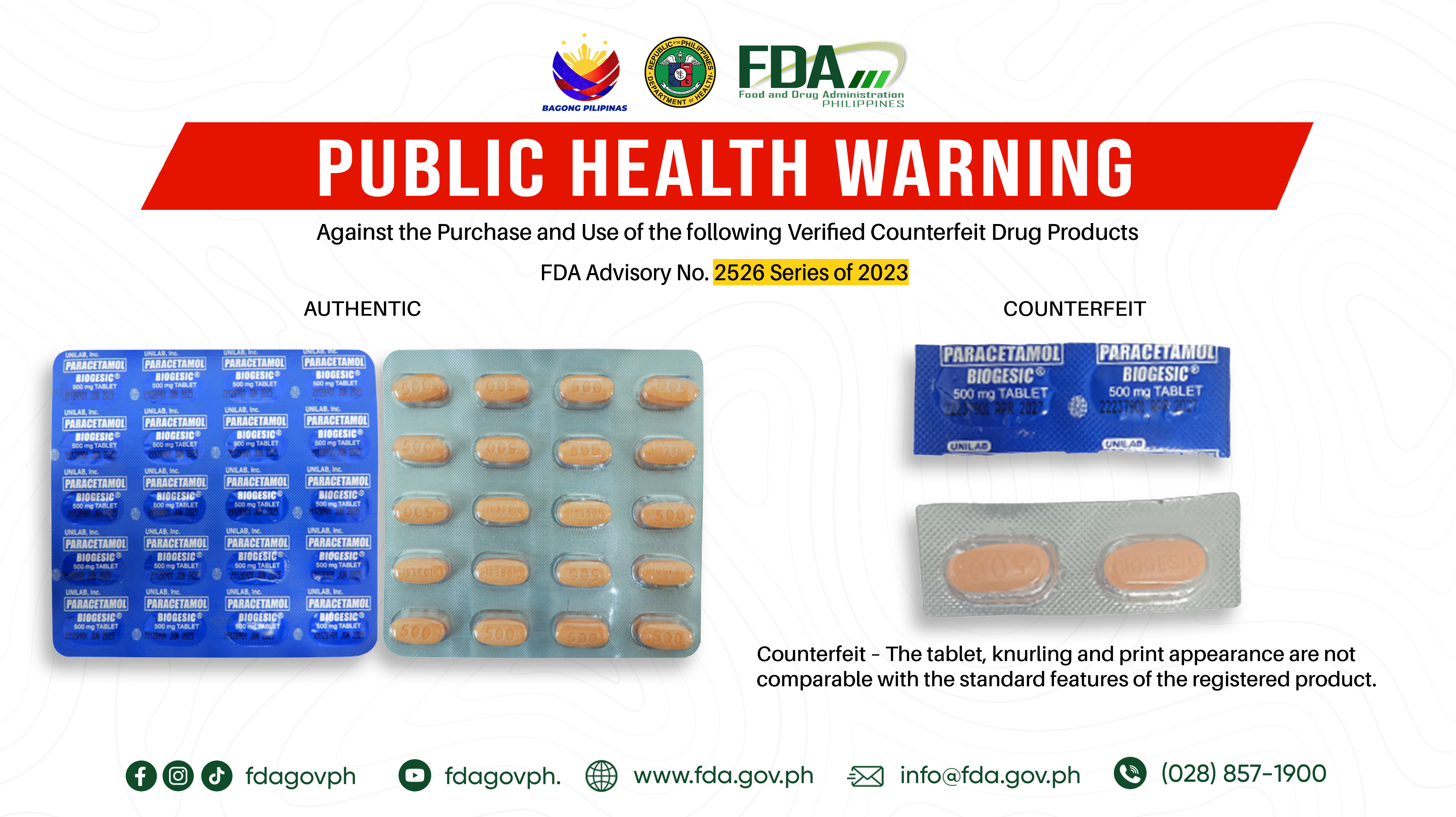
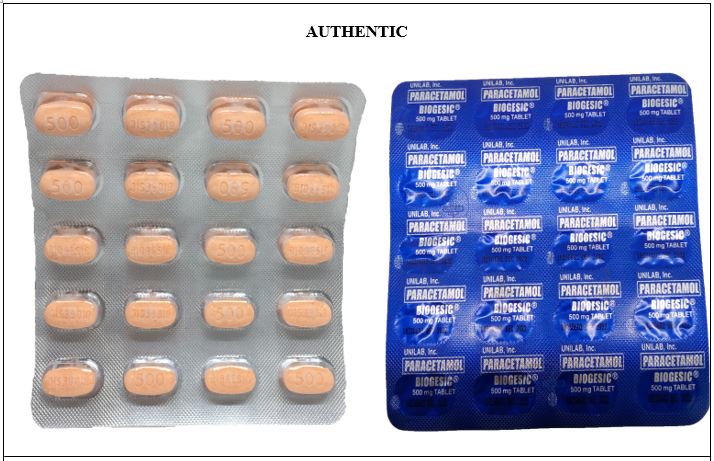
FDA Advisory No.2022-0135 || Public Health Warning Against the Purchase and Use of the Counterfeit Drug Product “Paracetamol (Biogesic®) 500 mg Tablet” - Food and Drug Administration

FDA Advisory No.2023-1838 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0779 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

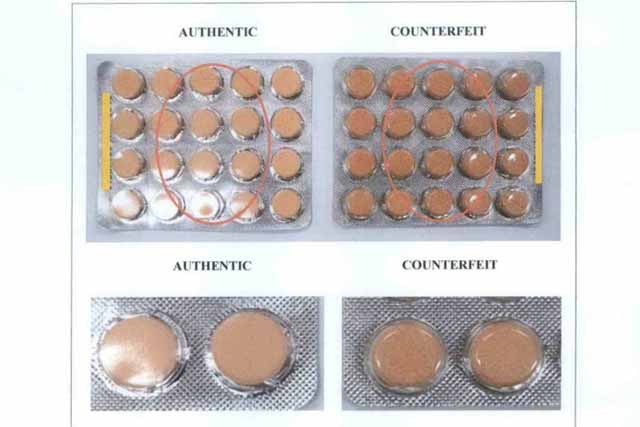
FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2023-1931 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
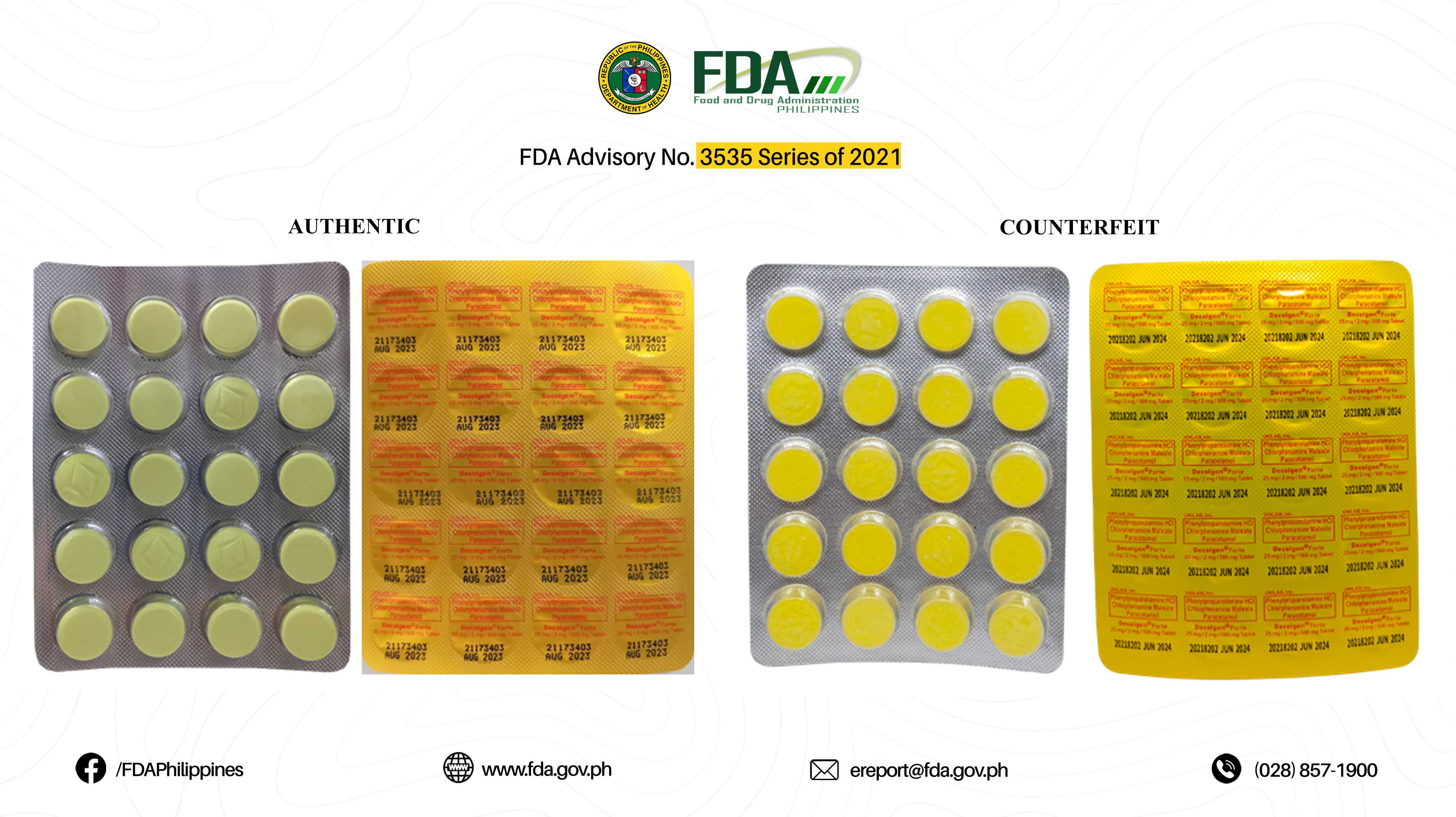
FDA Advisory No.2021-3535 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Approved Drugs Western Medicine Paracetamol Injection - China Drugs, Paracetamol | Made-in-China.com
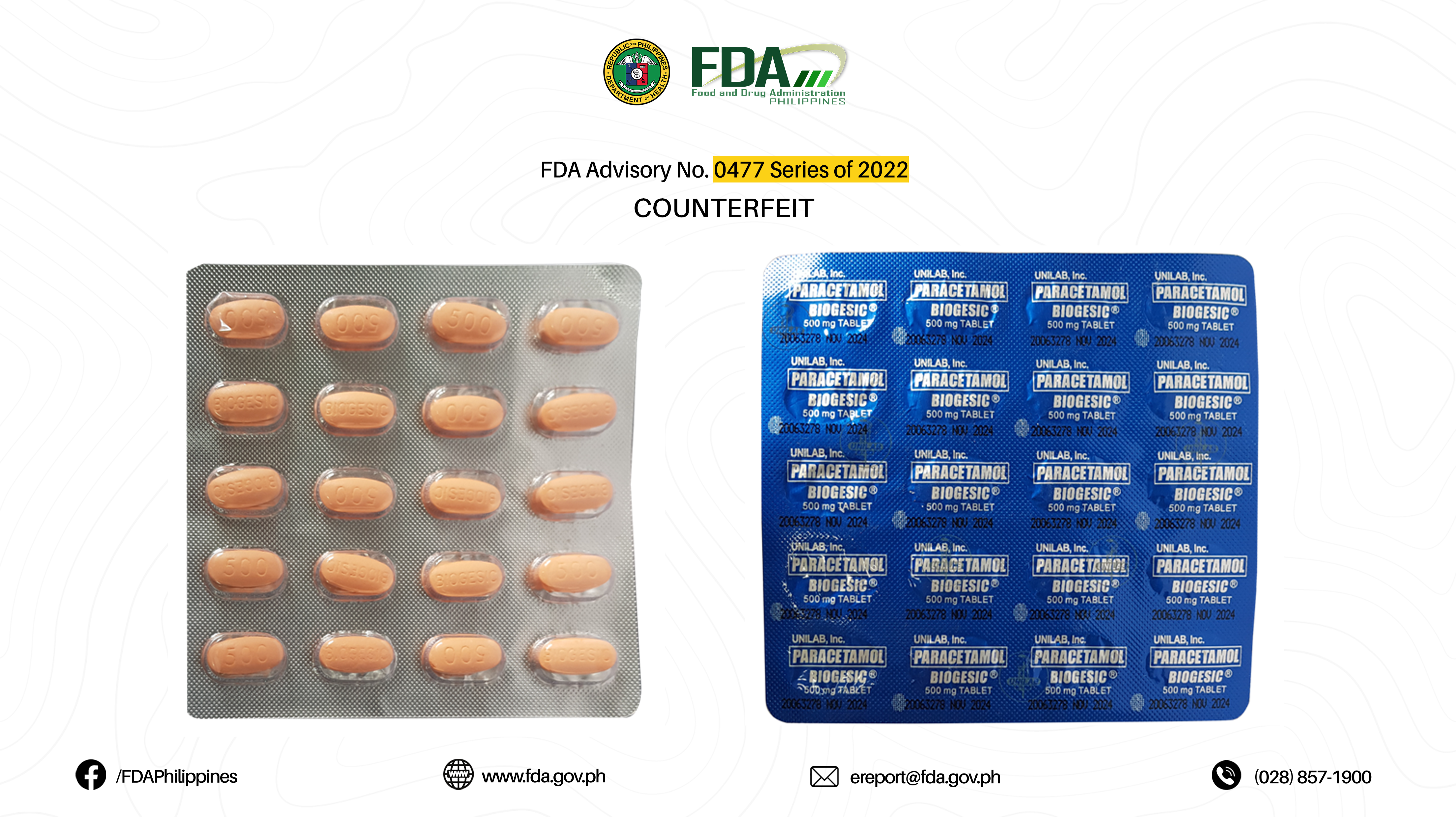
FDA Advisory No.2022-0477 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products - Food and Drug Administration

FDA Advisory No.2022-0620 || Public Health Warning Against the Purchase and Use of the Counterfeit Drug Product “Ibuprofen/ Paracetamol (Alaxan® FR) 200 mg / 325 mg Capsule” - Food and Drug Administration

FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2021-1723 || Public Health Warning Against the Purchase and Use of the Unregistered Drug Product “Perfalgan® Paracetamol 1 g in 100 mL Solution for Infusion 100 mL Vial” - Food and Drug Administration

FDA Advisory No.2023-1841 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0780 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration
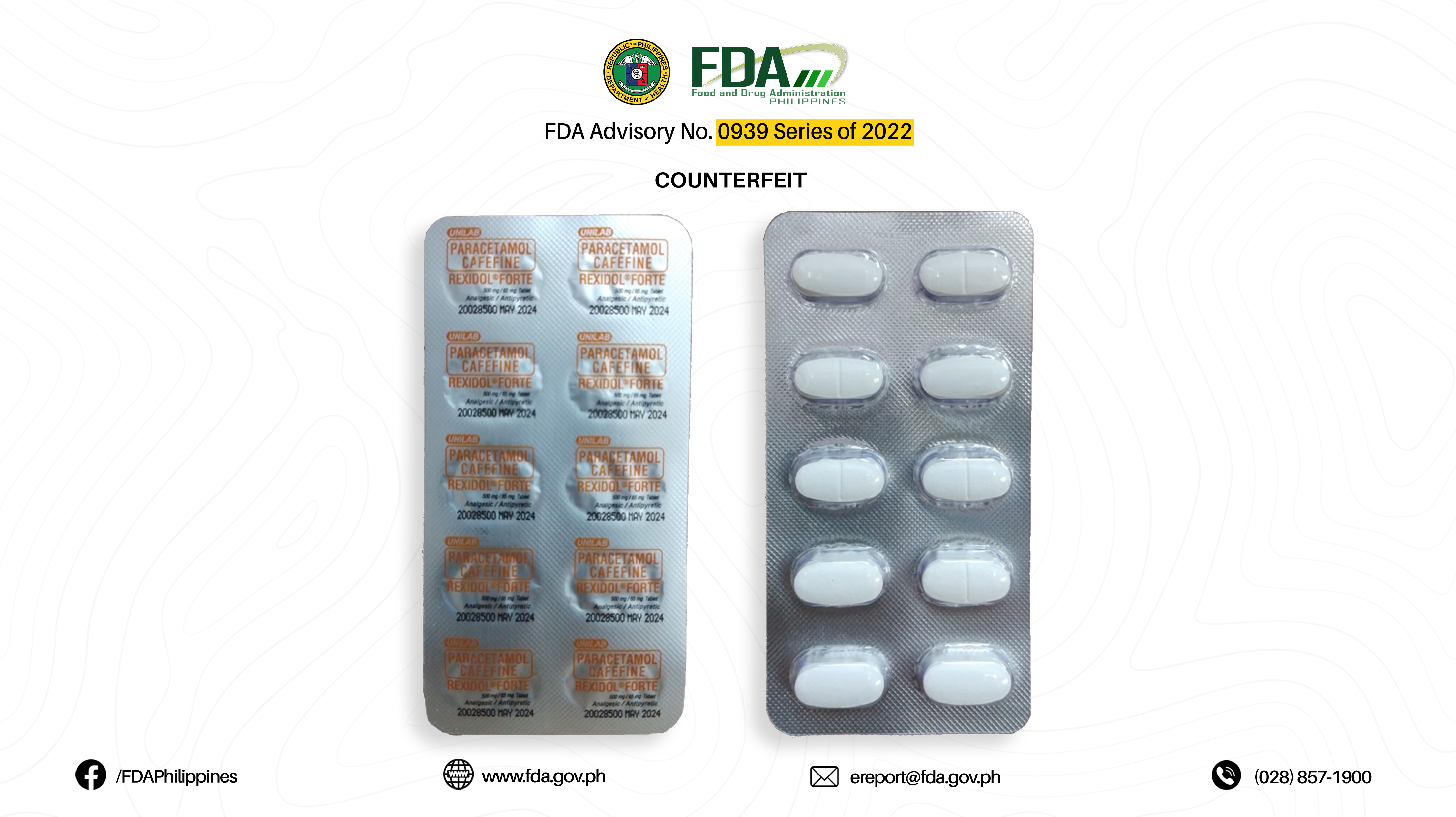
FDA Advisory No.2022-0939 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2023-1840 || Public Health Warning on Substandard (Contaminated) Paracetamol + Phenylephrine Chlorhydrate + Chlorpheniramine Maleate Syrup Confirmed by the World Health Organization (WHO) - Food and Drug Administration

FDA Advisory No.2023-2327 || Public Health Warning Against the Purchase and Use of the following Verified Counterfeit Drug Products: - Food and Drug Administration

FDA Advisory No.2022-0277 || Public Health Warning Against the Purchase and Use of the Verified Counterfeit Drug Product : - Food and Drug Administration