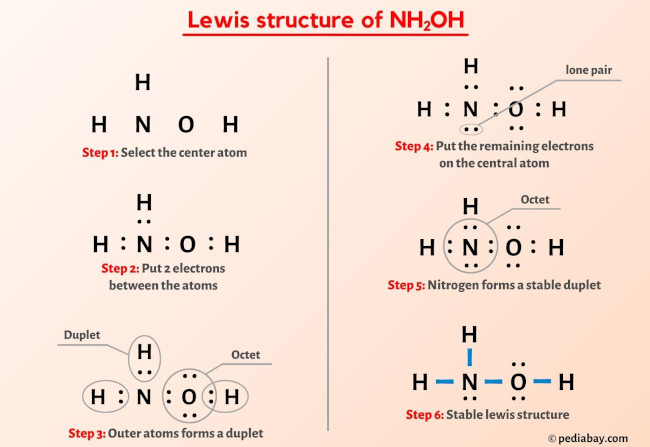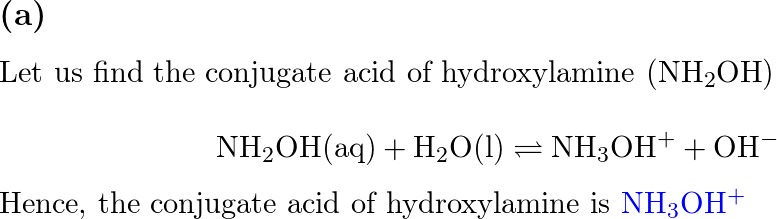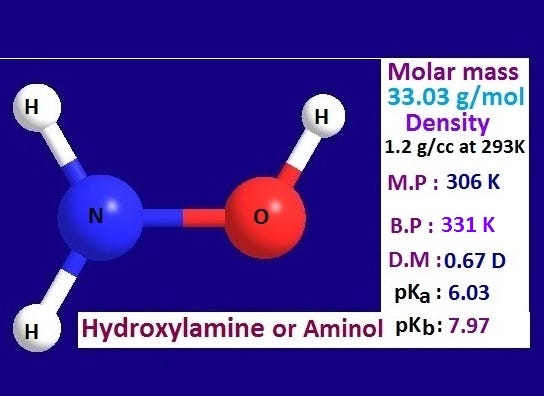
What is hydroxylamine?. Hydroxylamine is a nitrogenous… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium

Calculate the pH of a 0.050 M solution of hydroxylamine, NH2OH. (Kb = 6.6 x 10-9) | Homework.Study.com
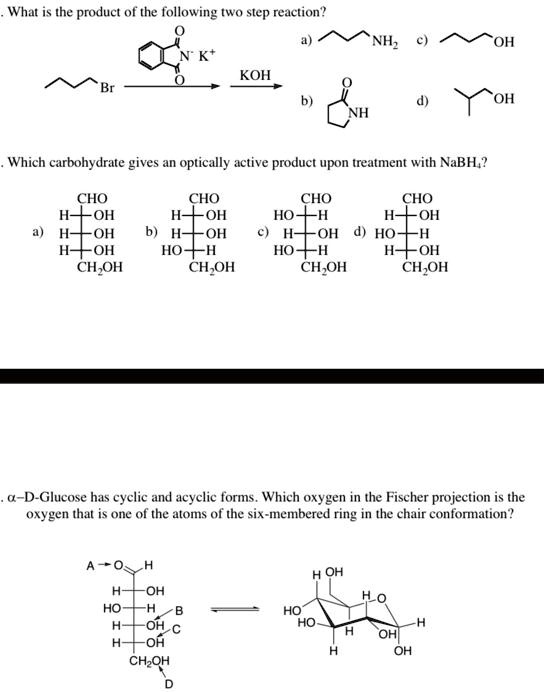
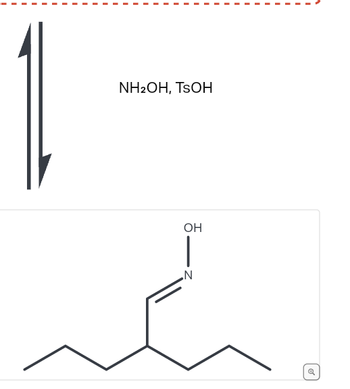
SOLVED: What is the product of the following two-step reaction? NH3 + KOH â†' NH2OH + H2O Which carbohydrate gives an optically active product upon treatment with NaBH4? CHO H-OH H-OH H-OH
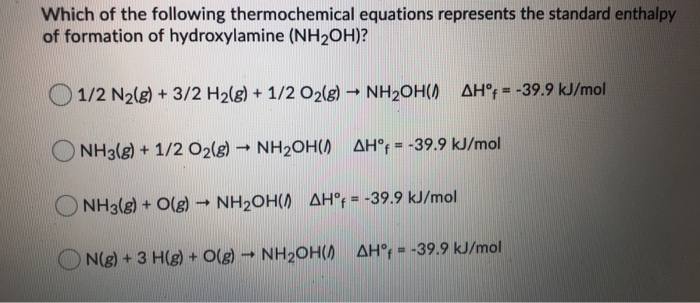
![PDF] Thermal decomposition of NH2OH and subsequent reactions: ab initio transition state theory and reflected shock tube experiments. | Semantic Scholar PDF] Thermal decomposition of NH2OH and subsequent reactions: ab initio transition state theory and reflected shock tube experiments. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b36782da9edaac7d0b91c7a6a1f8caff67f9661e/16-Figure22-1.png)
PDF] Thermal decomposition of NH2OH and subsequent reactions: ab initio transition state theory and reflected shock tube experiments. | Semantic Scholar

Figure 3 from Formation of hydroxylamine (NH2OH) in electron-irradiated ammonia-water ices. | Semantic Scholar

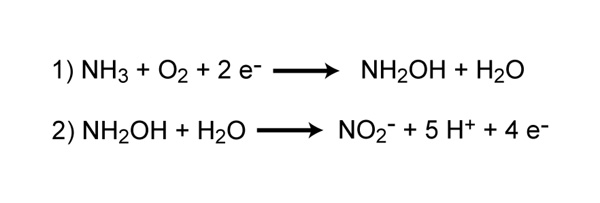
Is there a pathway for N2O production from hydroxylamine oxidoreductase in ammonia-oxidizing bacteria? | PNAS

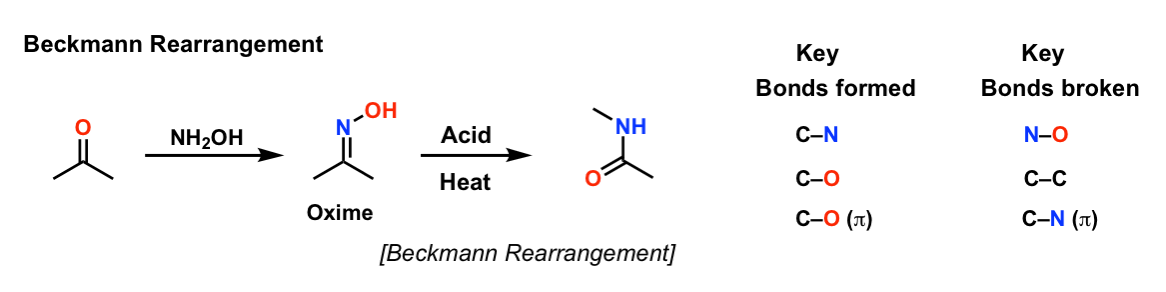
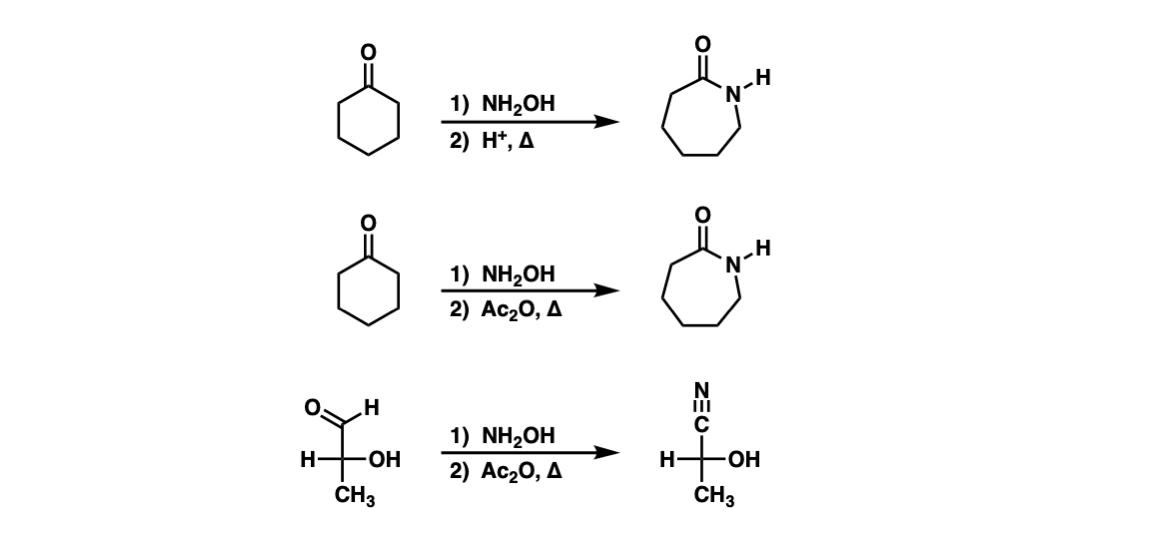
Combination of NH2OH·HCl and NaIO4: a new and mild oxidizing agent for selective oxidation of alcohols to carbonyl compounds - ScienceDirect
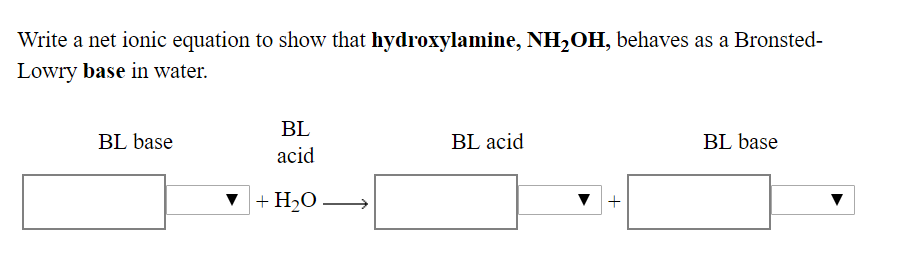
![SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-] SOLVED: 5. Hydroxylamine, NH2OH, reacts with water: NH2OH + H2O -> HNHOH* + OH- a) Is it acting as an acid or as a base? b) Identify its conjugate. c) The [OH-]](https://cdn.numerade.com/ask_images/db627e0c62e74e1a9a6bc5925a1a5d0a.jpg)