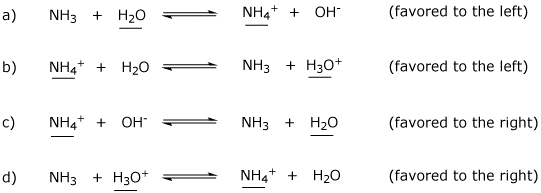
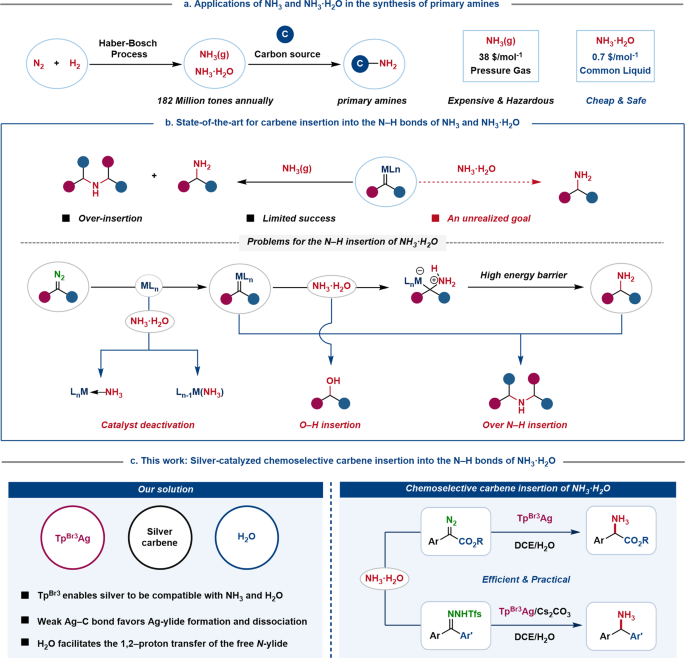
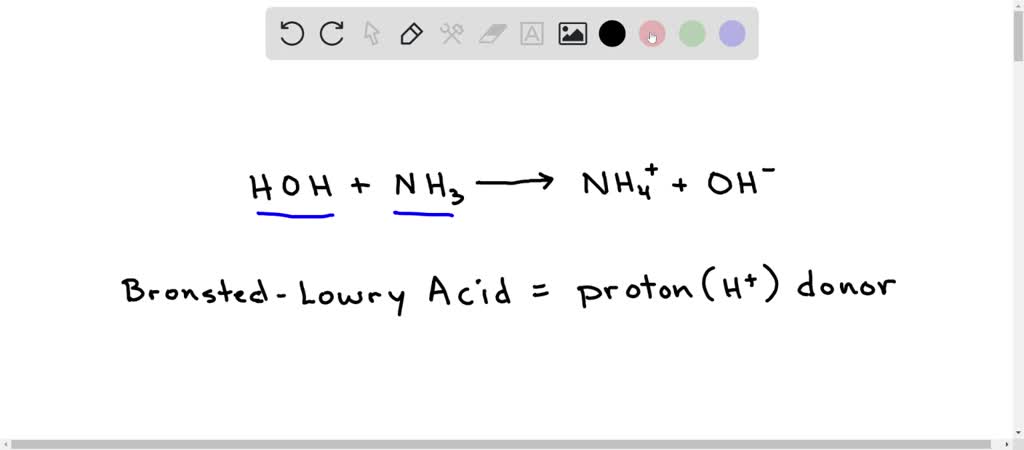
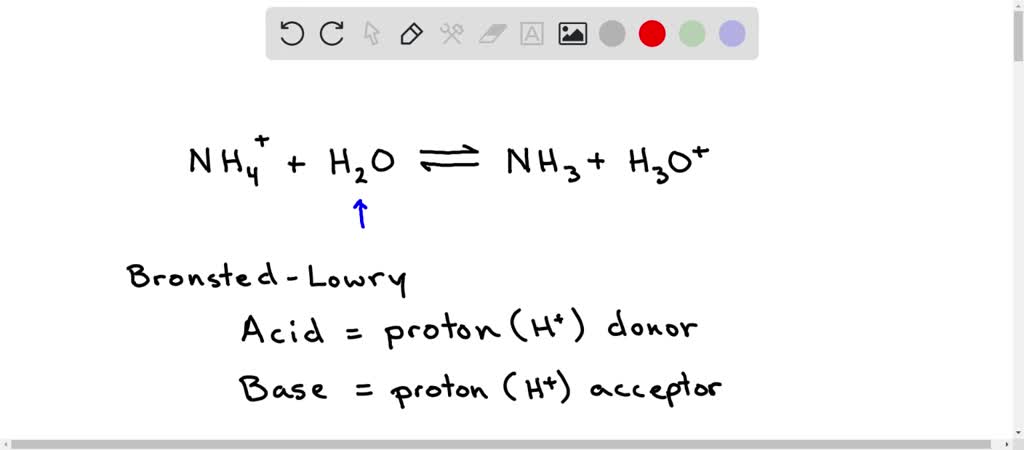
H,0(1) + NH3 (aq)OH (aq) + NH+ (aq) H2O(1)+H,S(aq)| H,O* (aq) +HS- (aq) The auto-protolysis (self-ionization) of water takes place as follows: H2O(1) + H2O(1) H,O* (aq) + OH- (aq) acid-1 base-2
Although the geometries of NH3 and H2O molecules are distorted, the tetrahedral bond angles in water is less than that of ammonia. Why? - Quora

Effect of water and ammonia on the HO + NH3 → NH2 + H2O reaction in troposphere: Competition between single and double hydrogen atom transfer pathways - ScienceDirect

Several isomers are possible for (Co(en)(NH3)(H2O)2Cl)2+. How many isomers can you draw for this complex? | Homework.Study.com
Catalytic effect of (H2O)n (n = 1–3) on the HO2 + NH2 → NH3 + 3O2 reaction under tropospheric conditions - RSC Advances (RSC Publishing)

Effect of water and ammonia on the HO + NH3 → NH2 + H2O reaction in troposphere: Competition between single and double hydrogen atom transfer pathways - ScienceDirect

Frontiers | Can a single ammonia and water molecule enhance the formation of methanimine under tropospheric conditions?: kinetics of •CH2NH2 + O2 (+ NH3/H2O)