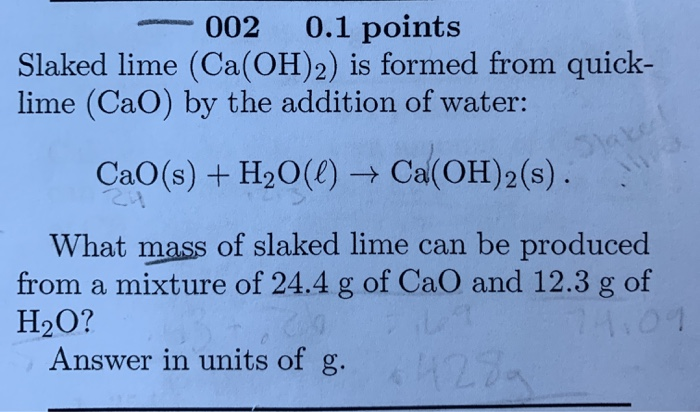Near-Infrared Spectroscopic Analysis─Formation of Ca(OH)2 and Ca(OD)2 by Hydration of CaO with H2O and D2O | The Journal of Physical Chemistry C

Insight into the Mechanism and Effect of H2O on CaO Sulfation by Density Functional Theory | Energy & Fuels

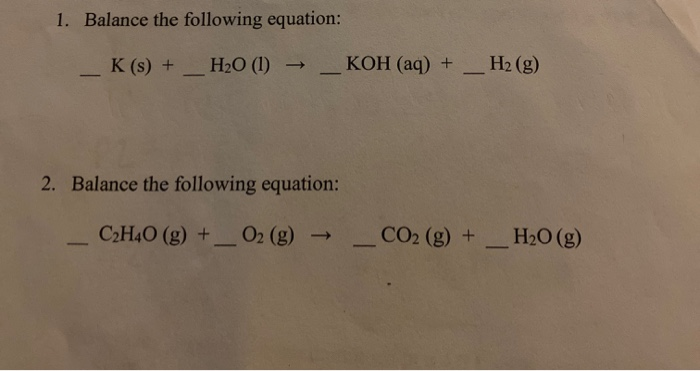
How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) | How to Balance Ca + H2O = Ca(OH)2 + H2 (Calcium plus Water) Hi Guys, welcome back to

chemmacros - What is wrong with \ch{CaO$_{(s)} + H2O$_{(l)}->Ca$^{2+}_{(aq)} + 2 OH$^-_{(aq)}} - TeX - LaTeX Stack Exchange

The mineralogy of the CaO–Al2O3–SiO2–H2O (CASH) hydroceramic system from 200 to 350 °C - ScienceDirect
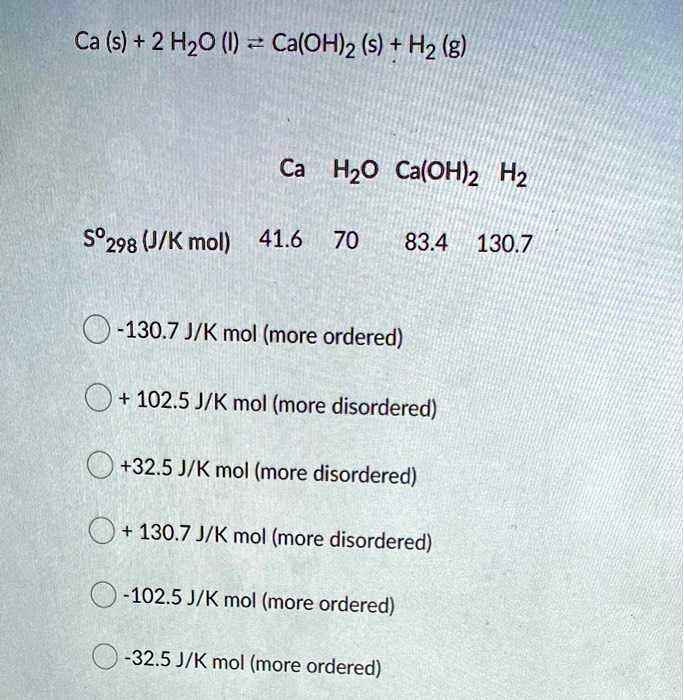
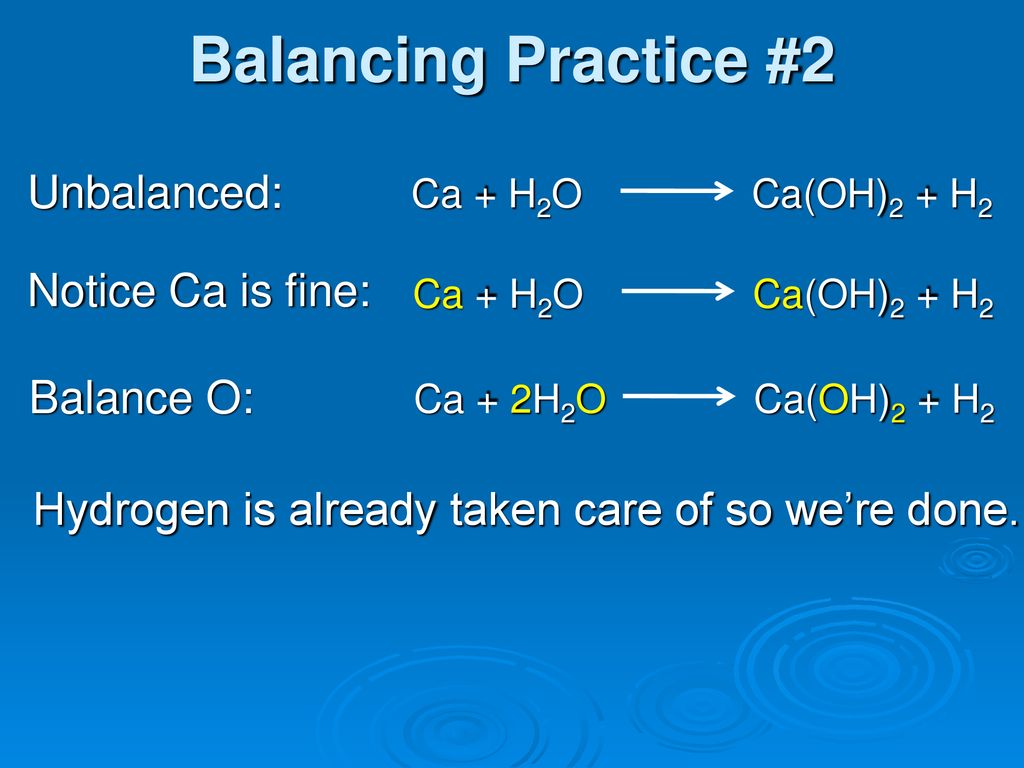
SOLVED: Ca (s) + 2 H2O (l) = Ca(OH)2 (s) + H2 (g) Ca H2O Ca(OH)2 H2 592.98 (J/K mol) 41.6 70 83.4 130.7 413.07 J/K mol (more ordered) 102.5 J/K mol (

1. The reaction between CaO and H2O is a) highly exothermic with hissing b) endothermic with hissing sound - Brainly.in

Please help with all parts and show work. The decomposition of Ca(OH)_2(s) into CaO(s) and H_2O(g) at constant pressure requires the addition of 109 kj of heat per mole of Ca(OH)_2. (a)

Are you confused with Uncertainty and Uncertainty? CaO -neglected lime (calcium oxide) Ca(OH)2.. | VK

Phase diagram for the system Ca(OH)2-CaO-O2.Free energy of the calcium... | Download Scientific Diagram