
Synthetic pathway of 1. a) NH2OH·HCl, BaCO3, Pd/C, N2H4·H2O, reflux in... | Download Scientific Diagram
Solved: The chemical equation here describes a reaction between barium carbonate and nitric acid. [algebra]

Cho sơ đồ các phản ứng theo đúng tỉ lệ mol:(a) X(t0) $ \to $ Y + CO2(b) Y + H2O $ \to $ Z(c) T + Z $ \to $ R + X + H2O(d?

SOLVED: Equation: Ba(OH)2(aq) CO2(g) BaCO3(s) H2O(l) Total ionic: Ba2+(aq) + 2OH-(aq) + CO2(g) â†' BaCO3(s) + H2O(l) Net ionic: Ba2+(aq) + CO2(g) â†' BaCO3(s) + H2O(l)

Vidéo de question : Identifier la substance qui contient à la fois des liaisons ioniques et des liaisons covalentes | Nagwa

Solve 53 sum 200 m' solution molarity of tho volumo ot CO, at S T P on heat'0 9 (2) - Chemistry - - 12898155 | Meritnation.com

Date twe of bor WYSICAL SUENCE Works Balance the following equations: 1. AL + N2 - AIN 2. Fe + 02 - Fe3O4 Caco - CaO + CO2 NH.NO, N2O + H2O


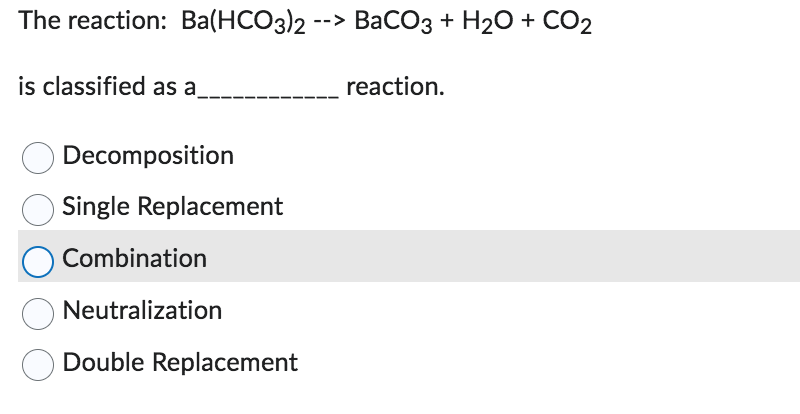




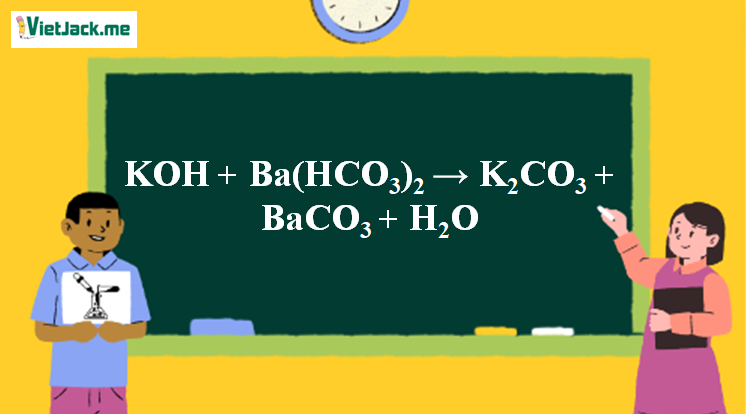
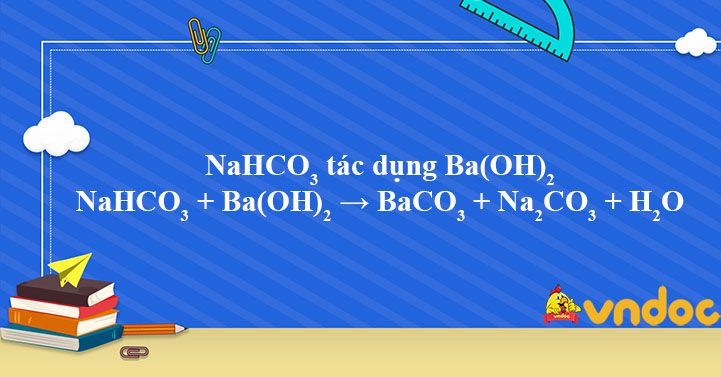
![ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry - Kunduz ANSWERED] BaCO3 + 2HNO3 → Ba(NO3)2 + CO₂ + H₂O What ... - Organic Chemistry - Kunduz](https://media.kunduz.com/media/answer/raw/20220422054128757632-4413054.jpg?type=wm)




