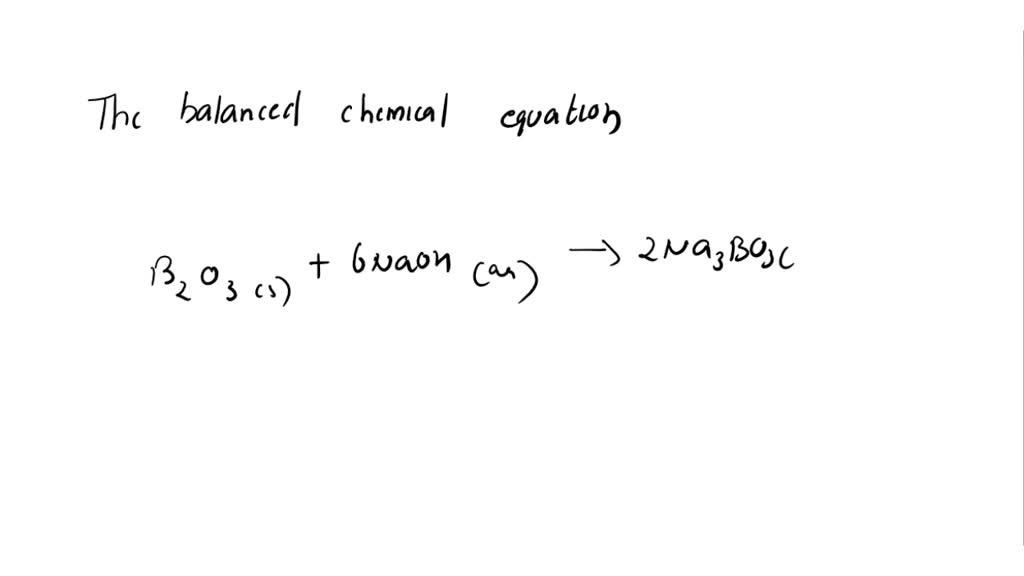
H3B03 on heating decomposes in two ways: 1. HBO3 + HBO2 + H2O II. H2B03 → B2O3 + H2O If 9 moles of H3BO3 are taken, some part decomposed like (1) and
![8-Diagramme d'équilibre de phase B2O3-SiO2 d'après Rockett et al. [RF65] | Download Scientific Diagram 8-Diagramme d'équilibre de phase B2O3-SiO2 d'après Rockett et al. [RF65] | Download Scientific Diagram](https://www.researchgate.net/publication/326029008/figure/fig12/AS:642325411807240@1530153720175/Diagramme-dequilibre-de-phase-B2O3-SiO2-dapres-Rockett-et-al-RF65.png)
8-Diagramme d'équilibre de phase B2O3-SiO2 d'après Rockett et al. [RF65] | Download Scientific Diagram

PDF) Phase equilibrium study in the CaO–K2O–B2O3–H2O system at 25°C | Laszlo Csetenyi - Academia.edu

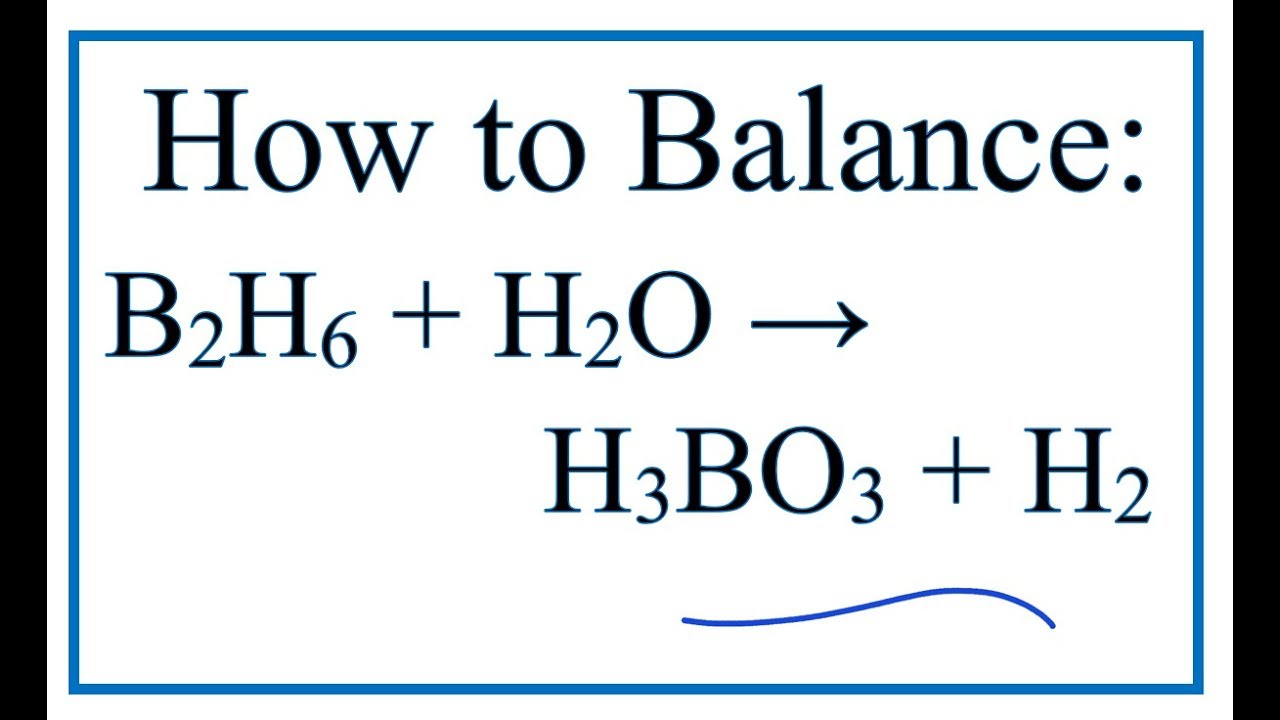
B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download

B2O3 + 3 H2O ---> 2 H3BO3 If diboron trioxide is reacted with water, the product is boric acid. What mass of boric acid is obtained from the full reaction. - ppt video online download
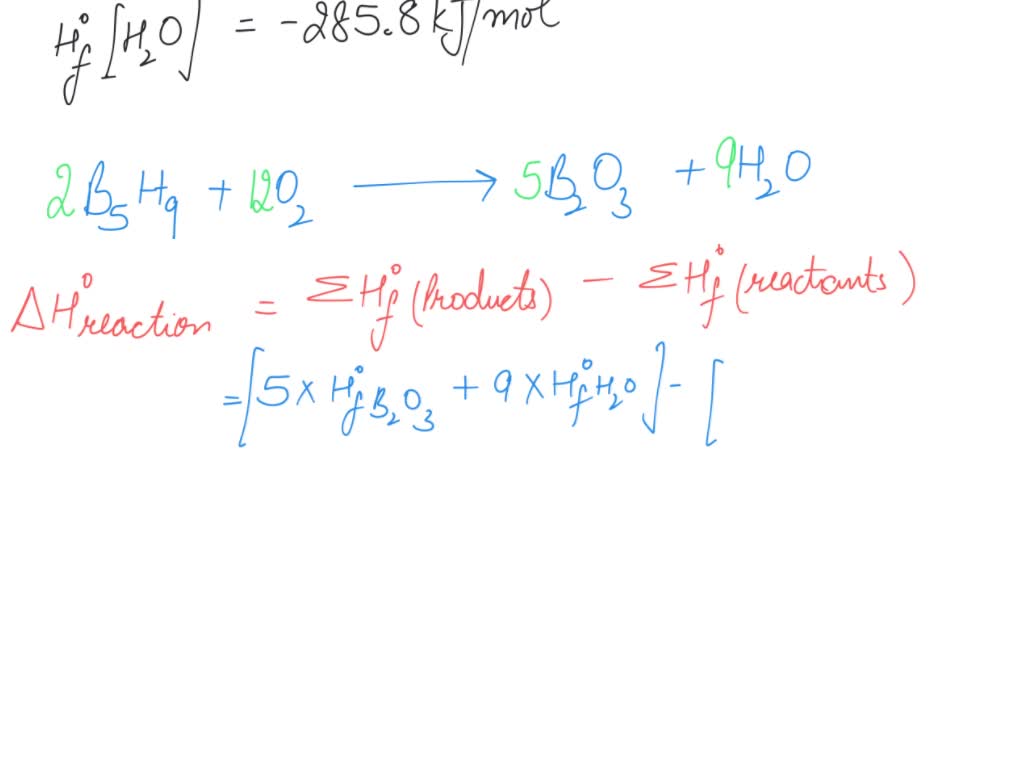
SOLVED: Pentaborane B5H9(s) burns vigorously in O2 to give B2O3(s) and H2O(l). What is ΔH° for the combustion of 1 mol of B5H9(s)? Substance ΔH°f (kJ/mol) B2O3(s) –1273.5 B5H9(s) +73.2 H2O(l) –285.8

Experimental and calculated phase diagrams in the system Na2O-B2O3-H2O... | Download Scientific Diagram
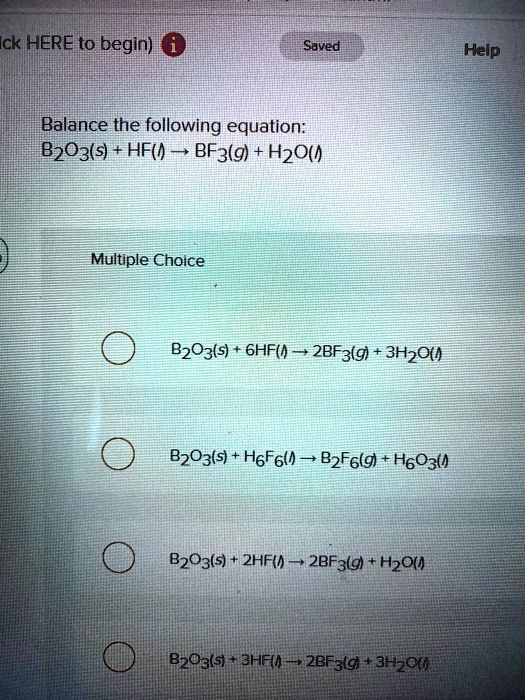
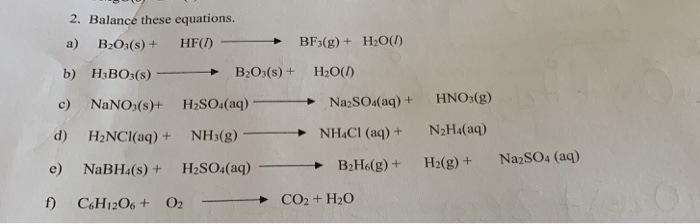
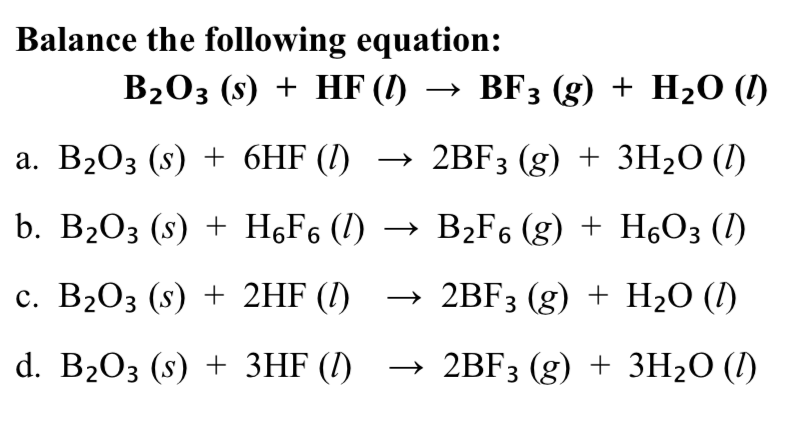
SOLVED: Click HERE to begin. Saved Help Balance the following equation: B2O3(s) + HF(g) â†' BF3(g) + H2O(l) Multiple Choice B2O3(s) + 6HF(g) â†' 2BF3(g) + 3H2O(l) B2O3(s) + 6HF(g) â†' B2F6(g) +